In yet another discovery we observe how the Spike Protein rapidly ages us. This time via the vasculature.
Atherosclerosis is normally a very gradual process.
Atherosclerosis develops slowly as cholesterol, fat, blood cells and other substances in your blood form plaque. When the plaque builds up, it causes your arteries to narrow. This reduces the supply of oxygen-rich blood to tissues of vital organs in the body.
What Is Atherosclerosis?
https://www.nhlbi.nih.gov/health/atherosclerosis
Yet, this process is also an INFLAMMATORY one of the Endothelium.
The atherogenic process starts with the accumulation of several plasma lipoproteins in the subendothelial space at sites of flow perturbation and endothelial dysfunction. This is best documented for LDL, whose accumulation correlates with classical risk factors, such as smoking, hypertension, and metabolic dysregulation in obesity and diabetes. In the intima, LDL undergoes oxidative modifications by reactive-oxygen species (ROS), which promote the uptake of oxLDL into macrophages. In addition, oxidized phospholipids per se trigger inflammation of the arterial wall by binding to Toll-like receptors (TLRs), a group of widely expressed pattern-recognition receptors (PRRs) that cause pro-inflammatory signaling. Clinically, oxLDL is a marker of plaque inflammation19. Native LDL can also be taken up by macrophages by micropinocytosis, or in its aggregated form as cholesterol complexes or -crystals by phagocytosis. The sustained influx of cholesterol eventually exceeds the phagocytes’ metabolic capacity and intracellular lipid droplets form. Microscopically, cholesterol-laden macrophages are ‘foam cells’.
Immunity and Inflammation in atherosclerosis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6342482/
Please note that the risk groups for the atherogenic process are the same as for COVID.
The Spike Protein mimics the effects of potentially DECADES of the atherogenic process virtually instantaneously.
Here we report that SARS-CoV-2 viral RNA is detectable and replicates in coronary lesions taken at autopsy from severe COVID-19 cases. SARS-CoV-2 targeted plaque macrophages and exhibited a stronger tropism for arterial lesions than adjacent perivascular fat, correlating with macrophage infiltration levels. SARS-CoV-2 entry was increased in cholesterol-loaded primary macrophages and dependent, in part, on neuropilin-1. SARS-CoV-2 induced a robust inflammatory response in cultured macrophages and human atherosclerotic vascular explants with secretion of cytokines known to trigger cardiovascular events. Our data establish that SARS-CoV-2 infects coronary vessels, inducing plaque inflammation that could trigger acute cardiovascular complications and increase the long-term cardiovascular risk.
SARS-CoV-2 infection triggers pro-atherogenic inflammatory responses in human coronary vessels
https://www.nature.com/articles/s44161-023-00336-5
And if you have any further doubts as to whether the Spike Protein is culpable:
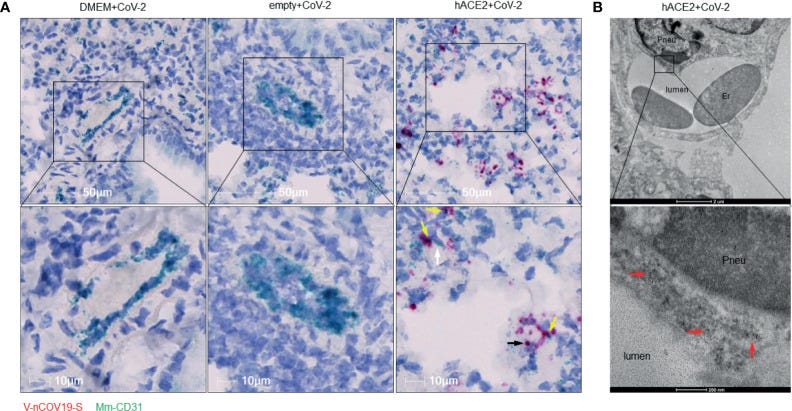
RNA of SARS-CoV-2 spike protein can be found inside endothelial cells. (A) Representative RNAscope images showed co-localization of RNA of spike protein with CD31 RNA in SARS-CoV-2 infected Ad5-hACE2 mouse lungs (right panel, n = 2), but not in lungs of control mice transduced by DMEM (left panel, n = 3) or Ad5-empty (middle panel, n = 3). White arrow: Spike RNA signals showing light green; black arrow: CD31 RNA signals showing red; yellow arrows: co-localization of two RNAs showing purple. (B) Immunoelectron microscope showed SARS-CoV-2 proteins (black particles indicated by red arrows, n = 1) inside endothelial cells of infected Ad5-hACE2 mice lungs. Pneu, pneumocyte; Er, erythrocyte.
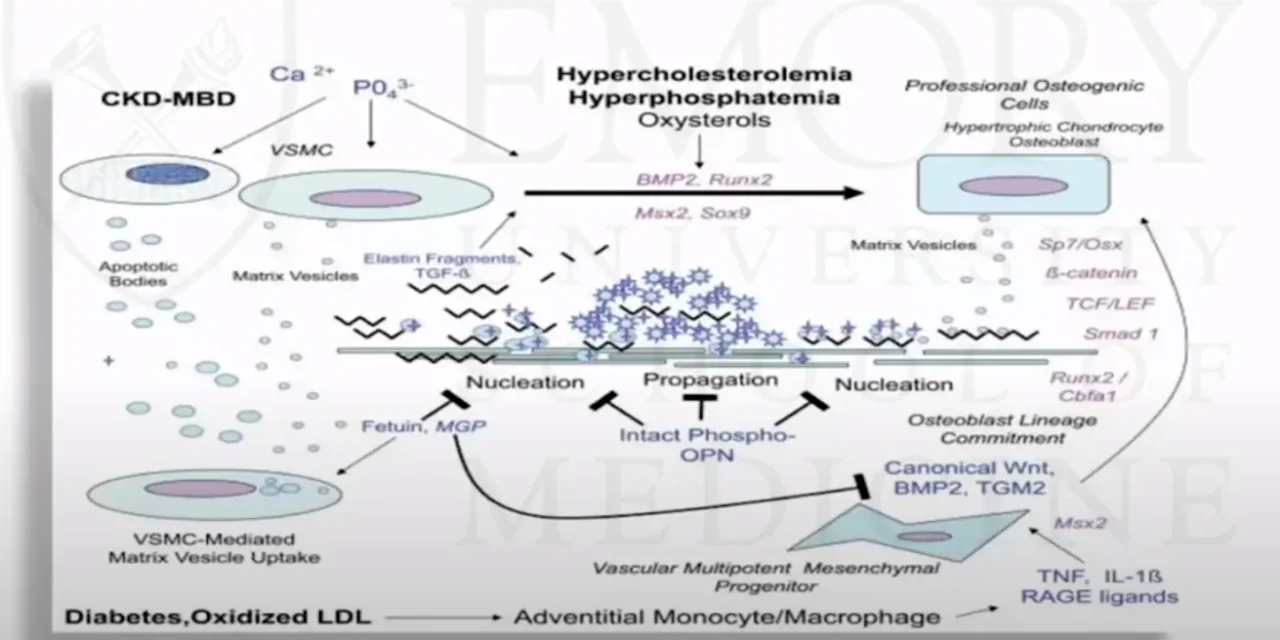
As if this were not bad enough, new research shows that this very pattern and type of damage can be osteogenic. It can turn the tissue to stone.
So, the pathobiology of internal calcification so internal calcification first starts off with atherosclerosis so atherosclerosis occurs before internal calcification and we know that atherosclerosis is an inflammatory process that starts with damage of the endothelium then monocytes enter the artery and differentiate into macrophages which proliferate locally they ingest oxidized lipids and then they slowly start to turn into large foamy macrophages and then they eventually die and which further propagates the inflammatory process in addition the smooth muscle cells start to proliferate and they migrate from the vessel wall in response to cytokines that is secreted by damaged endothelium cells and by the dying cells and they help and these smooth muscle cells are thought to help form the fibrous cap then the calcification starts to occur and internal calcification really resembles endochondral bone formation and although the initiation of the calcification does not require participation by specific cells but the progression of the lesion is thought to be likely driven by chondrocyte-like cells and associated with expression of inflammatory factors like cytokines…
Coronary Artery Calcification: When Artery Becomes Bone
And, yes, this happens:
The biopsy specimen showed a subcutaneous pauci-inflammatory thrombogenic vasculopathy accompanied by endothelial and mural calcification in microvessels.
The skin as a critical window in unveiling the pathophysiologic principles of COVID-19
https://www.sciencedirect.com/science/article/pii/S0738081X21001310
The more I study this virus and its Spike Protein, the more I am convinced that ACE2, as a receptor, is perhaps the most diabolical receptor a virus can use to cause both rapid and gradual decline in the human body.